How Patient Support Programs Can Compliantly Interact with Commercial Teams

Don joined Team Archbow this year, focusing on supporting quality patient access and care. Learn more about Don here.
What Recent Increased Compliance Requirements Mean for Pharmaceutical Manufacturers’ Patient Support Programs
By Don Hribek
Background
Considering the complexities associated with reimbursement and how HUBs can offset those complexities, it is no surprise that patient support programs are becoming more valuable to providers and patients. Although practices may manage some tasks internally and rely on external resources for odd payers or complex cases, there is no denying that the need for dedicated patient support programs continues to grow.
Moving Forward
Change is rarely easy. Eliminating all direct interactions between patient support program agents and sales representatives/commercial teams is especially difficult but necessary, in today’s environment. Patient support personnel and commercial field representatives must keep their roles and responsibilities separate, both internally and during interactions with HCPs.
The question remains: for patients currently prescribed a company product by an HCP and enrolled in the patient support program, what information can be shared with the commercial sales team? The answer will entirely depend on each company’s legal, privacy, and compliance professionals to determine the level of detail that can be shared.
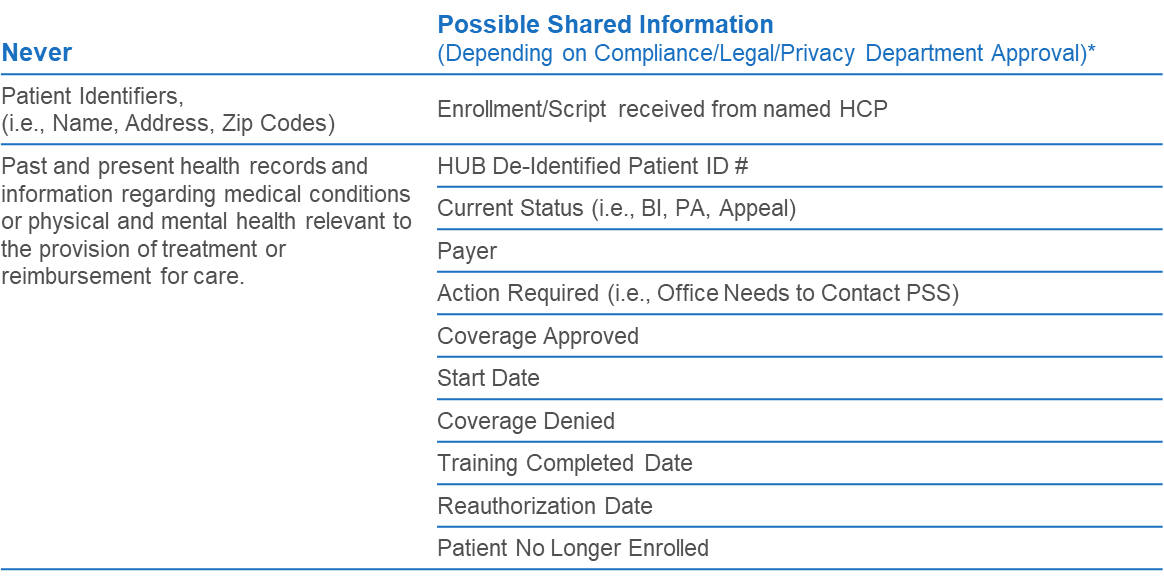
What does all of this mean for manufacturers?
It is now crucial that manufacturers ensure patient support programs are truly patient-focused and appropriately utilized to support the patients who are prescribed a manufacturer’s product. Many commercial teams may benefit from a reminder that patient support programs may not be used to induce, influence, or reward prescribing behavior, or create demand for, sell, or promote any product, service, or program.
Creating good standard operating practices/procedures with well-defined, specific guidelines coupled with ongoing training and certification of commercial and sales teams is essential. If your organization would like to better understand how to mitigate the potential negative impacts of compliance, we can help. Our pharmaceutical consulting company team guides manufacturers through the patient support design/redesign process, drafts SOPs, develops training, and implements program launches and modifications. Contact us today to get started.
For additional insights on this topic, you may also enjoy the following blog posts:
Archbow Consulting helps pharmaceutical and biotech companies in the USA and Europe design, build, and optimize product distribution and patient access strategies. Archbow was founded by industry veterans to meet a need in the marketplace for consulting options that offer diverse real-world experience, are able to leverage deep connections across the industry, and can also provide actionable strategic guidance. We invite you to learn more about our team, services, and clients’ success, and connect with us via email, LinkedIn or subscribing to this blog which you can do below.
Share in
Recent Posts
Self-Injection Training & Patient Success
Practical, hands-on self-injection training plays a pivotal role in ensuring proper medication use and driving positive patient outcomes.
Navigating Pharmacy Types for Commercialization Success
The pharmaceutical market is evolving fast, and delivering prescriptions efficiently is more challenging than ever. The right pharmacy dispensing strategy can be the difference maker between commercial success and missed opportunities.
DTC/DTP + Telemedicine – The Next Big Thing in Pharma?
The May 2025 Executive Order marks a seismic shift in the U.S. pharmaceutical patient access market.
SUBSCRIBE
Subscribe to receive news and updates from Archbow Consulting
|
|


